Publication – Understanding the performance of a microfluidic electrolysis cell for routine organic electrosynthesis
J. Flow. Chem, 2014
Robert A. Green, Richard C.D. Brown and Derek Pletcher
Chemistry Department, University of Southampton, Southampton, SO17 1BJ, UK
This paper aims to close the knowledge gap between microfluidic synthetic organic chemistry and optimized microfluidic organic electrosynthesis. This paper describes the optimization of a previously characterized anodic methoxylation reaction in respect to charge efficiency, selectivity, conversion and product formation rate with the fractional conversion rate being dramatically increased. It is concluded that the Electrochemical cell allows synthesis of several grams per hour with good selectivity.
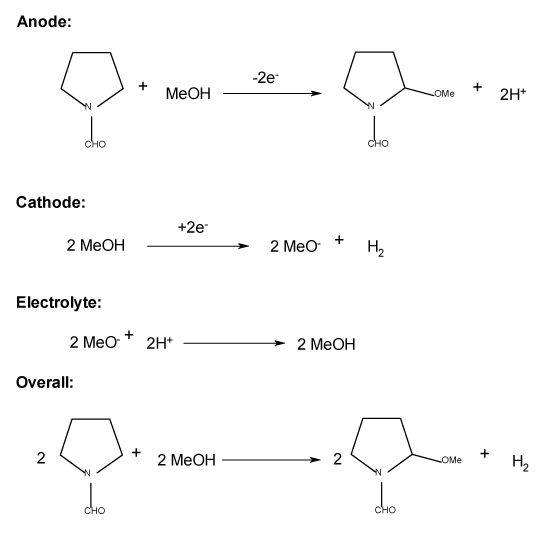
Abstract: In order for microflow electrolysis cells to make their full contribution to routine laboratory organic synthesis, they must be capable of carrying out reactions with good selectivity and high conversion at a high rate of conversion. In addition to the appropriate choice of the electrolysis medium and control of the overall cell chemistry, both the design of the electrolysis cell (including materials of construction) and the correct selection of the cell current and flow rate of the solution are critical in determining performance. The conclusions are tested using the methoxylation of N-formylpyrrolidine as the test reaction in a microflow electrolysis cell with a single, long, patterned flow channel.
This paper uses the Asia Flux. Learn more about the product this chemistry was performed on.
For more information, contact us.

