Publication – Synthesis of 4 substituted phthalazin-1(2H) ones from 2 acylbenzoic acids
Organic Process Research & Development 2015, 19 (7), pp 884-891
Steven M. Mennen, Melody L. Mak-Jurkauskas, Matthew M. Bio, L. Steven Hollis, Kelly A. Nadeau, Andrew M. Clausen and Karl B. Hansen
Process Development and Attribute Sciences, Amgen, Inc., Massachusetts, United States
Using the Syrris Atlas Calorimetry system, researchers in the Process Development and Attribute Sciences groups at Amgen (US) have improved on a key process for controlling the levels of Hydrazine in a Pharmaceutical Intermediate characterized using Power Compensation Calorimetry (PCC).
The group used PCC in tandem with in-situ IR techniques to understand the kinetics and thermodynamics of the two-step, one-pot chemical process. This was then used to develop their Pilot Plant Campaign.
A robust crystallization process was shown providing a critical control point ensuring high product quality and was successfully demonstrated on a multikilogram scale.
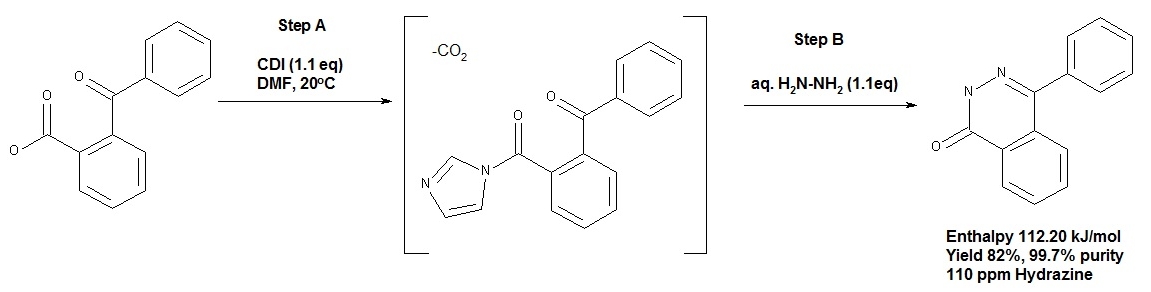
Abstract: A simple one-pot, two-step process for the conversion of 2-acylbenzoic acids to phthalazin-1(2H)-ones was developed. A robust process was required that delivered the final isolated solid with consistently low levels of residual hydrazine, for further processing to the final drug substance. An in situ formed intermediate was critical to control reactivity and allowed for the controlled crystallization that prevented entrainment of hydrazine. Leveraging Process Analytical Technology (PAT), we investigated the reaction profile with in situ IR and Power Compensation Calorimetry (PCC) to aid development prior to a successful scale-up.
This paper uses the Atlas Calorimeter. Learn more about the product this chemistry was performed on.
For more information, contact us.
